Nut Carcinoma of Maxillary Sinus – A Rare Entity
Divyamol N T1, Satheesh S1, Sindhu V Nath1, Susan James1, Aravindh S Anand2
- Department of Otorhinolaryngology, Government Medical College, Thiruvananthapuram;
2. Department of Radiotherapy, Government Medical College, Thiruvananthapuram*
Corresponding Author: Dr Divyamol N T
Junior Resident, Department of Otorhinolaryngology, Government Medical College, Thiruvananthapuram
Email: divyamolnt@gmail.com
ABSTRACT
Objective: To report a rare case of NUT carcinoma of maxillary sinus
Case Report: 55-year-old lady presented with left sided facial swelling for two months duration associated with facial pain for one month. After diagnostic nasal endoscopy and radiological evaluation, we proceeded with left middle meatal antrostomy and biopsy. Histopathology and IHC showed poorly differentiated NUT carcinoma. NUT carcinoma is a rare aggressive tumour with early regional and distant metastasis. At present there are no definite treatment guidelines available.
Keywords: Nuclear protein in testis (NUT), NUT carcinoma, Maxillary sinus, Poorly differentiated carcinoma
INTRODUCTION
Nuclear protein in testis (NUT) carcinoma is a rare, aggressive tumor defined by the presence of NUT gene rearrangement.1,2 Previously known as “NUT midline carcinoma” because most cases were found in midline of body, such as thorax or head and neck, it can arise in non-midline sites as well. It affects all age groups without sex predilection, although the median age is in the teens and young adulthood.3 Most NUT carcinomas harbor reciprocal translocation between NUT gene on chromosome 15q14 and BRD4 gene on chr19p13.4 Overall prognosis is poor with median survival ranging from 6.5 to 9.7 months. Mortality is usually due to rapid local progression and early distant metastasis. Studies show that head and neck NUT carcinoma has slightly better prognosis than thoracic variants. At present there are no definite treatment guidelines for this unique condition. Aggressive surgical resection with chemoradiation/radiation is the current treatment modality for head and neck NUT carcinoma.5,6
CASE REPORT
55-year-old lady presented with left sided facial swelling for two months and left sided facial pain for one month (Figure 1). On examination, there was a hard, tense, tender swelling of size 8x8 cm on left malar region with blunting of infraorbital margin. Skin over the swelling was non pinchable with no local rise in temperature and hypoesthesia. Lesion extended superiorly up to left lower eyelid, inferiorly to superior alveolar process, medially till left nasofacial groove and laterally up to malar prominence. Vision and extraocular movements were normal. Intraoral examination revealed a smooth bulge over the hard palate on left side. No cervical lymphadenopathy was noted.
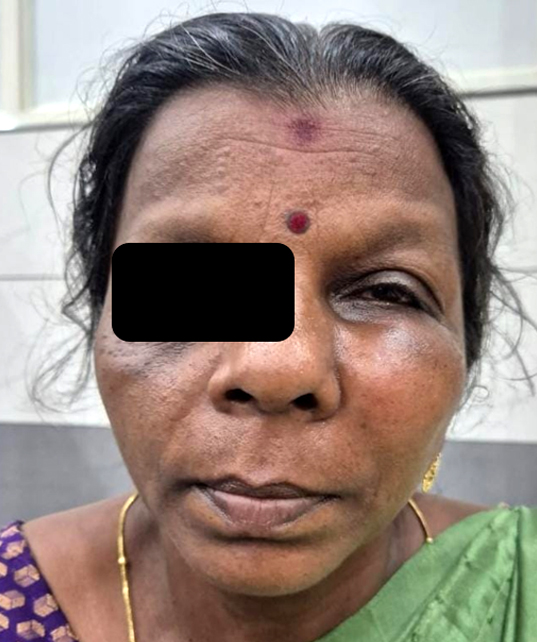
Figure 1: Left facial swelling
Diagnostic nasal endoscopy showed bulge in left lateral wall obliterating middle meatus with blood stained mucoid discharge in nasal cavity. Contrast enhanced CT and MRI scan of brain (Figure 3) showed heterogeneously enhancing lesion in left maxillary sinus with extension into orbit, nasal cavity, infratemporal fossa, superior alveolar process of maxilla, inferior orbital fissure and infiltration of maxillary division of trigeminal nerve with extensive bone destruction (Figure 2).
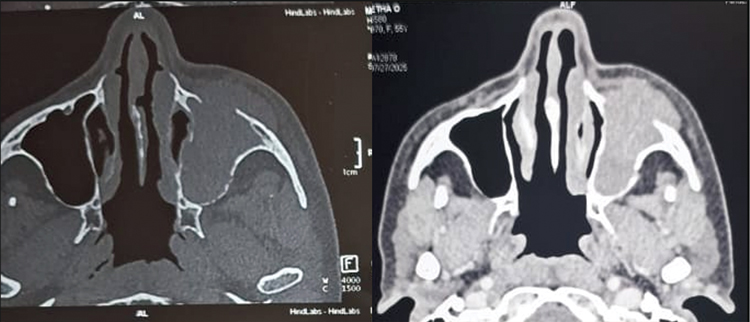
Figure 2. Contrast enhanced CT Nose and PNS
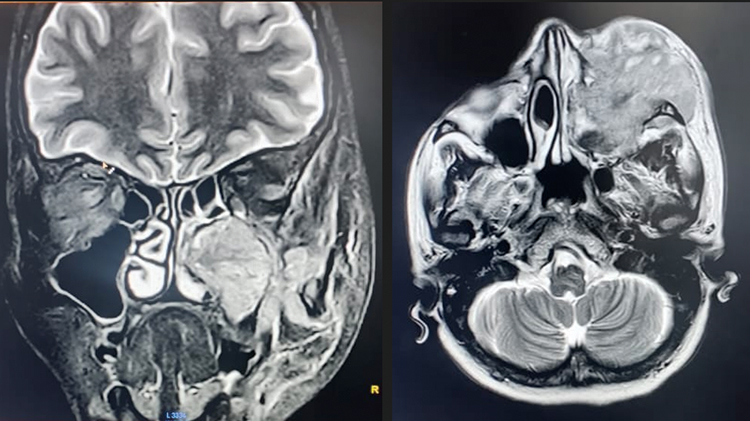
Figure 3. Contrast enhanced MRI brain
We proceeded with left middle meatal antrostomy and biopsy and sufficient tissue was sent for HPE. Initial histopathological examination revealed necrotic tissue with scanty atypical cells showing hyperchromatic nuclei, and a repeat biopsy was advised. Within a short interval, the lesion showed rapid progression in size, raising suspicion of a high-grade sarcoma or hemorrhage. Hence, repeat biopsy was done and histopathological examination showed infiltrative poorly differentiated carcinoma. Immunohistochemical analysis demonstrated positivity for Pan-CK, p63, and p40, confirming the diagnosis of a focal keratinizing poorly differentiated NUT carcinoma. The patient was referred to Radiation oncology and commenced on a cisplatin and 5-fluorouracil-based chemotherapeutic regimen. Following the initial cycle of chemotherapy, the patient demonstrated transient symptomatic improvement. However, despite continuation of systemic therapy, there was evidence of rapid progression of disease. In view of the aggressive course, palliative radiotherapy is planned if there is inadequate response to the subsequent chemotherapy cycle.
DISCUSSION
NUT carcinoma was initially described as a malignant tumor of the mediastinum and thymus.7 However, it has recently been reported in other parts of the body, including the stomach, kidney,8 eye,9 and pancreas.10 Its incidence in the head and neck region is approximately one-third to a half of all NUT carcinomas, and the nasopharynx accounts for more than half of all cases in the head and neck region.11 The parotid gland,12 submandibular gland,13 larynx,14 hypopharynx15 and palatine tonsils16 are the other sites that have been reported. NUT carcinoma is an aggressive malignancy with median survival of less than 1 year and presents as a rapidly enlarging mass with 33% regional lymph node metastasis and 13% distant metastasis.17 Multiorgan dissemination occurs later in the disease course.
Due to the lack of disease-specific histology and the diversity of the disease, it is often diagnosed as poorly differentiated or undifferentiated carcinoma.13,18 Diagnosis by conventional pathological examination alone is difficult. Although chromosomal analysis can confirm the diagnosis, it is not routinely feasible due to cost and time constraints. In recent years, immunohistochemical diagnosis using a monoclonal antibody against NUT carcinoma has become a useful alternative to chromosomal testing. Immunohistochemistry using a monoclonal NUT antibody has recently emerged as a reliable alternative for diagnosis.19 It is therefore essential to include NUT IHC testing in all undifferentiated or poorly differentiated head and neck carcinomas to prevent misdiagnosis and facilitate early intervention.
Gross total resection of the primary tumour is significantly associated with prolonged survival. As NUT carcinomas have rapid and aggressive growth, patients usually present with unresectable tumors or metastatic disease and surgical resection is not feasible in most cases. There are a few reports of patients who have benefited from chemotherapy and radiation therapy without surgery.
CONCLUSION
NUT carcinoma of the maxillary sinus is an exceptionally rare and only few cases are reported in world literature. It is an aggressive neoplasm with a poor prognosis due to its rapid progression and early metastatic potential. Diagnosis is often delayed because of its nonspecific clinical and histopathological features. Immunohistochemical detection of NUT protein is essential for accurate and timely diagnosis. Multimodal management incorporating surgery, chemoradiation, and emerging targeted agents may offer potential survival benefit in selected patients.
END NOTE
Author information
- Dr Divyamol N T, Junior Resident,
Department of Otorhinolaryngology,
Government Medical College, Thiruvananthapuram
Email: divyamolnt@gmail.com - Dr Satheesh S, Professor & HOD,
Department of Otorhinolaryngology
Government Medical College, Thiruvananthapuram
Email: shereensatheesh@yahoo.co.in - Dr Sindhu V Nath, Assistant Professor
Department of Otorhinolaryngology
Government Medical College, Thiruvananthapuram
Email: sindhuvn@gmail.com - Dr Susan James, Associate Professor
Department of Otorhinolaryngology
Government Medical College, Thiruvananthapuram
Email: drsusanjames@gmail.com - Dr Aravindh S Anand, Professor & HOD
Department of Radiotherapy,
Government Medical College, Thiruvananthapuram
Email: anandrt2006@yahoo.com
Financial Support: Nil
Conflict of Interest: None declared
REFERENCE
- Stevens TM, Morlote D, Xiu J, Swensen J, Brandwein-Weber M, Miettinen MM, et al. NUTM1-rearranged neoplasia: a multi-institution experience yields novel fusion partners and expands the histologic spectrum. Mod Pathol. 2019;32(6):764–773.
[Pubmed] - French CA, Kutok JL, Faquin WC, Toretsky JA, Antonescu CR, Griffin CA, et al. Midline carcinoma of children and young adults with NUT rearrangement. J Clin Oncol. 2004;22(20):4135–4139.
[Pubmed] - Bauer DE, Mitchell CM, Strait KM, Lathan CS, Stelow EB, Luer SC, et al. Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin Cancer Res. 2012;18(20):5773–5779.
[Pubmed] - French CA, Miyoshi I, Kubonishi I, Grier H, Perez-Atayde A, Fletcher JA.
BRD4-NUT fusion oncogene: a novel mechanism in aggressive carcinoma. Cancer Res. 2003;63(2):304-307.
[PubMed] - French CA. NUT carcinoma: clinicopathologic features, pathogenesis, and treatment. Pathol Int. 2018;68(11):583-595.
[PubMed] - Chau NG, Hurwitz S, Mitchell CM, Aserlind A, Grunfeld N, Kaplan L, et al. Intensive treatment and survival outcomes in NUT midline carcinoma of the head and neck. Cancer. 2016;122(23):3632-3640.
[PubMed] - Kubonishi I, Takehara N, Iwata J, Sonobe H, Ohtsuki Y, Abe T, Miyoshi I. Novel t(15;19)(q15;p13) chromosome abnormality in a thymic carcinoma. Cancer Res. 1991;51(12):3327-3328.
[PubMed] - Dickson BC, Sung YS, Rosenblum MK, Reuter VE, Harb M, Wunder JS, et al. NUTM1 gene fusions characterize a subset of undifferentiated soft tissue and visceral tumors. Am J Surg Pathol. 2018;42(5):636-645.
[PubMed] - French CA, Kutok JL, Faquin WC, Toretsky JA, Antonescu CR, Griffin CA, Nose V, Vargas SO, Moschovi M, Tzortzatou-Stathopoulou F, Miyoshi I, Perez-Atayde AR, Aster JC, Fletcher JA. Midline carcinoma of children and young adults with NUT rearrangement. J Clin Oncol. 2004;22(20):4135-4139.
[PubMed] - Shehata BM, Steelman CK, Abramowsky CR, Olson TA, French CA, Saxe DF, et al. NUT midline carcinoma in a newborn with multiorgan disseminated tumor and a 2-year-old with a pancreatic/hepatic primary. Pediatr Dev Pathol. 2010;13(6):481-485.
[PubMed] - French CA. NUT carcinoma: clinicopathologic features, pathogenesis, and treatment. Pathol Int. 2018;68(11):583-595.
[PubMed] - Lee T, Cho J, Baek CH, Son YI, Jeong HS, Chung MK, et al. Prevalence of NUT carcinoma in head and neck: analysis of 362 cases with literature review. Head Neck. 2020;42(5):924-938.
[PubMed] - Bishop JA, Westra WH. NUT midline carcinomas of the sinonasal tract. Am J Surg Pathol. 2012;36(8):1216-1221.
[PubMed] - French C, Miyoshi I, Kubonishi I, Grier H, Perez-Atayde A, Fletcher JA. BRD4-NUT fusion oncogene: a novel mechanism in aggressive carcinoma. Cancer Res. (2003) 63(2):304.
- French CA, Rahman S, Walsh EM, Kuhnle S, Grayson AR, Lemieux ME, et al. NSD3-NUT fusion oncoprotein in NUT midline carcinoma: implications for a novel oncogenic mechanism. Cancer Discov. 2014;4(8):928-941.
[PubMed] - Grayson AR, Walsh EM, Cameron MJ, Godec J, Ashworth T, Ambrose JM, et al. MYC, a downstream target of BRD-NUT, is necessary and sufficient for the blockade of differentiation in NUT midline carcinoma. Oncogene. 2014;33(13):1736-1742.
[PubMed] - Chau NG, Hurwitz S, Mitchell CM, Aserlind A, Grunfeld N, Kaplan L, Hsi P, Bauer DE, Lathan CS, Rodriguez-Galindo C, Tishler RB, Haddad RI, Sallan SE, Bradner JE, French CA. Intensive treatment and survival outcomes in NUT midline carcinoma (NMC) of the head and neck (HN). Cancer. 2016 Dec 1;122(23):3632–3640.
- Solomon LW, Magliocca KR, Cohen C, Müller S. Retrospective analysis of nuclear protein in testis (NUT) midline carcinoma in the upper aerodigestive tract and mediastinum. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119(2):213-220.
[PubMed] - Haack H, Johnson LA, Fry CJ, Crosby K, Polakiewicz RD, Stelow EB, et al. Diagnosis of NUT midline carcinoma using a NUT-specific monoclonal antibody. Am J Surg Pathol. 2009;33(7):984-991.
[PubMed]